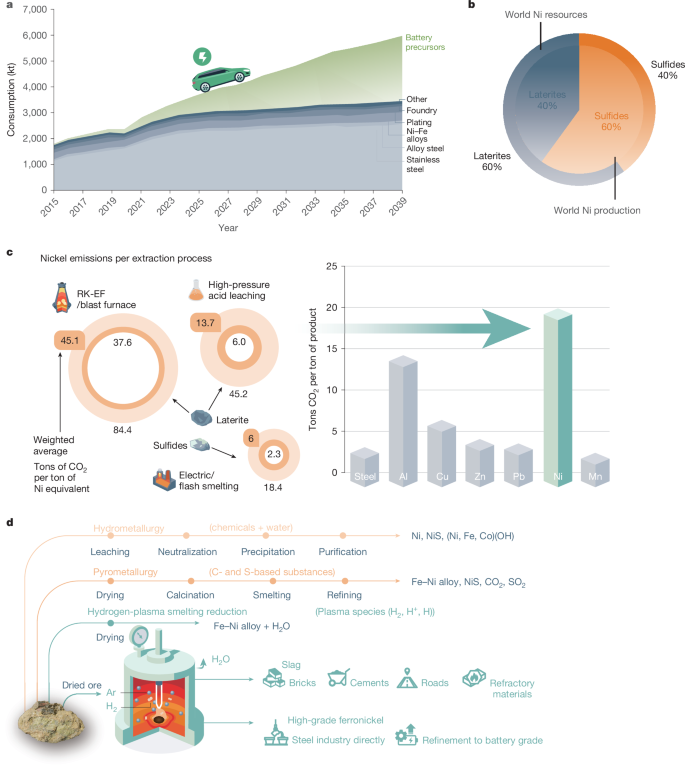
Nickel (Ni) is a strategic and hard-to-replace element used in 1.97 million tons of stainless steel and 210 kilotons of non-ferrous alloys, especially superalloys, annually1,2,3. Both alloy families serve sustainability indirectly, the former through enhanced longevity of products and the latter through higher efficiency of engines. Although 70% of the current global annual Ni production (3 million tons)1,2,3 is destined for the stainless-steel sector, the decarbonization of the transport sector through the use of Ni-based battery electrodes in electric vehicles is forecast to require an additional 3 million tons of Ni solely for battery production by 2040. This will cause a doubling of the global Ni demand to 6 million tons per year1,2,3,4 (Fig. 1a). Currently, 60% of the annual Ni production relies on high-grade sulfide ores (with 1.5–4 wt% Ni content). Low-grade ore variants, namely, laterites (with an average 1.5 wt% Ni content), which are subdivided into two variants, namely, saprolite and limonite, provide the remaining 40% (ref. 8; Fig. 1b). However, land-based Ni reserves are inversely distributed, that is, 60% of the total Ni available in nature is found in laterites, and only 40% in sulfide ores8 (Fig. 1b).
The market growth, sources, production, processing routes, emissions and comparison with the sustainable one-step hydrogen-plasma route. a, Current and projected (2040) Ni-market growth, fuelled by surging demand for battery electrode precursor materials needed for the electrification of the transport sector, propelling Ni demand to double from 3 million tons to 6 million tons (data from ref. 1), at a staggering environmental toll of 20 tons of CO2 per ton of Ni (10 times the emission caused by the production of a ton of steel). b, Schematic showing the land-based world Ni-resources distribution and their corresponding share in global Ni supply (data from ref. 8). c, CO2 emissions (tons per ton of Ni) from various extraction routes. The inner and outer circles signify lower and upper emission limits. The highlighted middle ring numbers depict weighted averages for each process (data from ref. 15). Bar plots on the right show the overall average CO2 emissions from the Ni industry (highlighted by green arrow), alongside comparisons with other industries (data from refs. 5,7). d, Comparative analysis of current processing routes and HPSR: the schematic diagram highlights distinct processing steps for each extraction method. One-step HPSR stands out as a single-step process, directly feeding dried ore into a furnace flooded with an Ar–H2 mixture. This streamlined process yields high-grade ferronickel, slag and gaseous water as the products.
In sulfidic ore deposits, Ni exists predominantly in the form of discrete binary and ternary Ni-rich minerals such as NiS, Ni2FeS4 and (Co,Ni)3S4. The chemical simplicity of sulfide minerals enables effective separation of gangue impurities from valuable Ni-bearing compounds using conventional techniques such as froth flotation9. However, the finite and declining reserves of Ni sulfides cannot meet the rapidly increasing global Ni demand, prompting the need for sustainable Ni production from abundant, low-grade laterites. In laterite deposits, Ni is not found as discrete minerals; it is dissolved within complex magnesium (Mg)-silicates (namely, saprolites)10, such as (Mg,Fe,Ni)3Si2O5(OH)4 and (Mg,Fe,Ni)3Si4O10(OH)2.4H2O, or partially replaces iron (Fe) in Fe-oxide lattices (limonite)7, namely, goethite (Fe,Ni)OOH. This mineralogical and chemical complexity limits the efficient and sustainable beneficiation of these ores into Ni-concentrated substances for downstream green technologies. This fundamental challenge motivated us to develop a completely different approach, namely, a smelting reduction process of the entire dried ore charge in a single metallurgical step using hydrogen plasma. This process integrates calcination, smelting and refining into a single step, with all these operations occurring simultaneously within one furnace, thus, allowing for the direct tapping of high-grade ferronickel from the dried ore charge in just one metallurgical step. A detailed explanation of the term ‘single metallurgical step’ is provided in Supplementary Information section ‘Definition of a single metallurgical step’.
The current industrial Ni-laterite processing routes are largely dictated by the crystallographic structure of Ni-hosting phases and the Ni and Fe content in the ore. Limonite ores, characterized by low Ni and MgO content (<4 wt% Mg), are typically processed via high-pressure acid leaching (HPAL) for Ni and cobalt (Co) recovery, when present11,12. In contrast, silicate-rich saprolites, which are difficult to reduce, undergo pyrometallurgical processing. This involves ore drying and partial reduction in rotary kilns (RK) using coke as a reductant, followed by smelting in electric arc furnaces (EAFs), a method known as RK-EF13,14, or alternatively in blast furnaces13. Further technical details are provided in Supplementary Information section ‘Current industrial processing routes of Ni-laterites’
The energy demand for laterite-ore processing is immense, ranging from 230 GJ per ton of Ni to about 570 GJ per ton of Ni (refs. 5,6), far exceeding the 22 GJ per ton required for steel. Greenhouse gas emissions are also substantial, with the RK-EF/blast furnace and HPAL routes emitting 45 tons of carbon dioxide equivalent (CO2e) per ton of Ni and 14 tons CO2e per ton of Ni, respectively15 (Fig. 1c). Although 60% of Ni comes from lower-emission sulfide processing (6 tons CO2e per ton of Ni)15, laterites account for 40%, pushing the industry’s overall footprint to about 20–27 tons CO2e per ton of Ni (refs. 5,6,7; Fig. 1c), over 10 times that of steel (2.3 tons CO2e per ton). This makes Ni one of the most environmentally harmful metals to produce (Fig. 1c).
To mitigate emissions, researchers have investigated replacing carbon-based reductants with hydrogen gas (H2)7 in the solid-state direct reduction of laterites, particularly limonite7,16,17. However, the complex crystallographic structure, low Ni content (0.5–2 wt%) and up to 90% impurity contents (oxides of silicon (Si), calcium (Ca) and aluminium (Al) to name a few) hinder reduction, leading to sluggish kinetics16,18 and inefficient hydrogen utilization18,19, and necessitates several additional pre- and post-treatments20 to separate the metal from unreduced oxides. The direct reduction of saprolites with gaseous hydrogen (H2) is scarcely reported, probably owing to the thermodynamic stability of silicates18,21 at typical direct-reduction temperatures (800–1,000 °C), and its reduction by molecular hydrogen might not be feasible without prior chemical transformation into simpler substances, such as NiO, by using catalyst compounds22, as detailed in Supplementary Information section ‘Solid-state DR of laterites’.
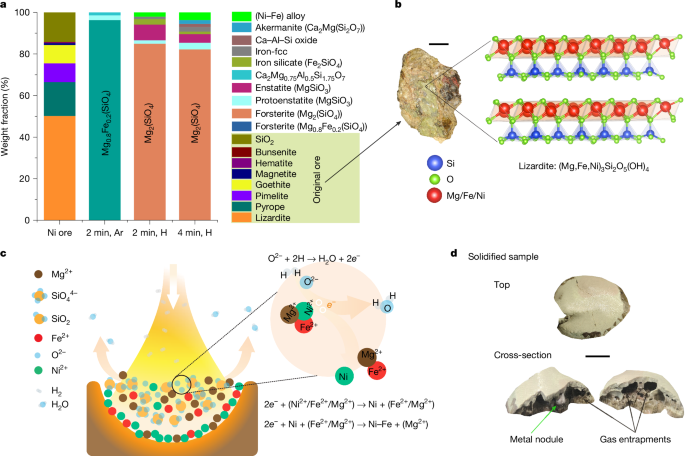
To overcome these limitations, particularly the ones associated with the saprolites, we subject the ore (Extended Data Tables 1 and 2) to a single-step hydrogen-plasma molten state process, thereby dissociating the crystallographic structure of the minerals (for example, (Mg,Fe,Ni)3Si2O5(OH)4; Fig. 2b) into simpler ionic species (Fe2+, Ni2+, Mg2+, O2− and SiO44−) without the need of catalysts, and concomitantly exposing it to the highly reactive hydrogen-plasma species (H2, H and H+). This approach consolidates the lengthy and environmentally harmful processes shown in Fig. 1 into a single metallurgical step: hydrogen-plasma smelting reduction (HPSR), beneficiation, metal separation and refining. Operable entirely on renewable energy, it replaces carbon-based fuels and reductants with renewable electricity and hydrogen, offering up to 18% energy savings and a reduction in CO2 emissions of up to 84%. Additional plasma-free experiments with molten Ni ore and molecular hydrogen were conducted as reference and comparison tests. The results showed significantly slower reaction kinetics with molecular hydrogen compared with hydrogen plasma, discussed in detail in Supplementary Information section ‘Experiments with molecular hydrogen’.
The complex Ni-hosting mineral structure in the original ore, reduction mechanisms and a visual snapshot of a solidified sample. a, Phase evolution during melting of the original ore in Ar and a lean H2 atmosphere (Ar–10% H2) for 2 min and 4 min, depicting the transformation of the complex original ore with various phases into simple Mg-silicate phases. The term ‘(Ni–Fe) alloy’ (shown in bright green), represents the metallic nodules, and ‘Iron-fcc’, shown in grey, denotes iron droplets entrapped in silicates that could not be mechanically recovered. b, Photograph of the original ore alongside the complex structure of lizardite that is present in it. Lizardite exhibits the capacity to host Ni2+/Fe2+ ions by replacing Mg2+ ions, highlighting the structural intricacies within the ore. c, Schematic hypothesis of the reduction mechanism. Precipitation of metals is initiated by the removal of free oxygen from the melt. Hydrogen species extract free oxygen, leaving behind two electrons. These electrons selectively react with Ni2+, inducing the precipitation of metallic Ni. The corresponding ionic reactions are also illustrated. d, Solidified sample and cross-section. Notably, metallic nodules are observable within the sample, alongside substantial porosities resulting from gas entrapments. Scale bars, 1 cm (b) and 5 mm (d).
Our investigation is focused on understanding the hydrogen-plasma reduction behaviour of saprolitic low-grade Ni laterite ore (Extended Data Tables 1 and 2). This approach marks a promising departure from conventional industrial techniques such as RK-EF/blast furnace and HPAL, by replacing carbon (C)- and sulfur (S)-based reducing agents with hydrogen and hence minimizing or eliminating direct CO2 and sulfur dioxide (SO2) emissions, circumventing the use of harmful acids (for example, sulfuric acid (H2SO4) in HPAL), and negating the need for costly pre- and post-treatments (Fig. 1d). This study provides experimental evidence supporting one-step HPSR as a sustainable alternative for metal production from both oxides and silicates. This expands feedstock options to low-cost, low-grade minerals. We investigate the thermodynamics, chemical partitioning, microstructural evolution and reaction mechanisms of Ni extraction from lateritic ores, applying these principles for precise and optimal process design.