
A participant in a study of cannabinol is monitored while sleeping.Credit: Woolcock Institute of Medical Research, Macquarie University
Miranda cannot remember a time in her life when she did not have insomnia. The 23 year old, who asked for her last name to be withheld, started struggling with sleep when she was a child. As she’s grown older, it’s only become worse. She takes “a myriad of medications” each night, she says, but usually still cannot fall asleep until the early hours of the morning. “I can’t get up and be functional until halfway through the day,” she says. She had to drop out of university because she couldn’t attend classes, and she can’t hold down a job. Her insomnia exacerbates other medical conditions as well, including migraines and the pain condition fibromyalgia. “It’s hugely debilitating,” she says. “It affects everything.”
In the United States, about 12% of adults have been diagnosed with chronic insomnia — when a person struggles to sleep for more than three nights each week for at least three months, and experiences daytime distress as a result. Research suggests that the worldwide figure is 10–30%. It also often co-occurs with and creates a vicious cycle with other conditions, including chronic pain, depression and anxiety.
Fortunately for Miranda and millions of others with chronic insomnia, new treatments are arriving. The emergence of a class of pharmaceuticals that induces sleep through a different brain pathway from existing drugs is a welcome development, and molecules in cannabis and specialized medical devices to promote sleep are also showing potential as sleep aids. Soon, those struggling with sleep could have a range of new options available to help.
Imperfect solutions
Cognitive behavioural therapy for insomnia (CBT-I) is usually the recommended first treatment. This specialized talking therapy focuses on establishing healthy sleep behaviours and addressing thoughts that can interfere with sleep. But CBT-I is not covered by all health-care insurance plans in the United States. In the United Kingdom and parts of Europe, public health-care systems usually provide it, but waiting times can be long. This is because, around the world, there is a limited availability of therapists, says Andrew Krystal, a psychiatrist at the University of California, San Francisco. “We keep hiring new people, but almost immediately their schedules are completely filled and the wait list is a year.”
CBT-I also doesn’t work for everyone. Miranda has tried it and has received conventional talking therapy for over a decade, with limited success. “It only helps so much,” she says.
Pharmacological interventions are the next line of defence, Krystal says. Benzodiazepines and a class of medicines called Z-drugs, which include zolpidem (Ambien), are among the most prescribed insomnia medications. These sedative hypnotics enhance the effects of the neurotransmitter GABA, thereby dampening brain activity. They also reduce anxiety. But they can create a hangover effect and increase the risk of falls in older people. These drugs also have the potential for misuse and can cause dependence. Some studies1 have even found an association between long-term use of Z-drugs and benzodiazepines and an increased risk of death.
Nature Outlook: Sleep
Miranda tried Ambien, but says that she quickly became chemically dependent. She eventually weaned herself off it and switched to benzodiazepines, but she began developing a tolerance to them, too — she once wound up in hospital with withdrawal symptoms after she tried to cut back on her dosage. “They’re horrible drugs to be on,” she says. But she cannot fall asleep without them. Each night, she now takes two benzodiazepines, as well as gabapentin, an anticonvulsant medication that is sometimes given off-label for insomnia.
Physicians frequently provide other off-label prescriptions for insomnia, including trazodone, which is approved for depression. Over-the-counter products such as antihistamines are also used for sleeplessness. None are ideal, however, because they have not been evaluated as sleep aids, says Emmanuel Mignot, a sleep-medicine researcher at Stanford University in California.
Miranda has experience with many of these products. When she first developed chronic insomnia as a child, her paediatrician recommended melatonin, which is available without a prescription in the United States. It helped her fall asleep, but it did not keep her asleep. During her teenage years, different neurologists prescribed off-label antidepressants and other mood medications, including trazodone and mirtazapine. But they came with what she calls “torturous” side effects: she felt constantly anxious and exhausted during the day, and her memory became “incredibly foggy”.
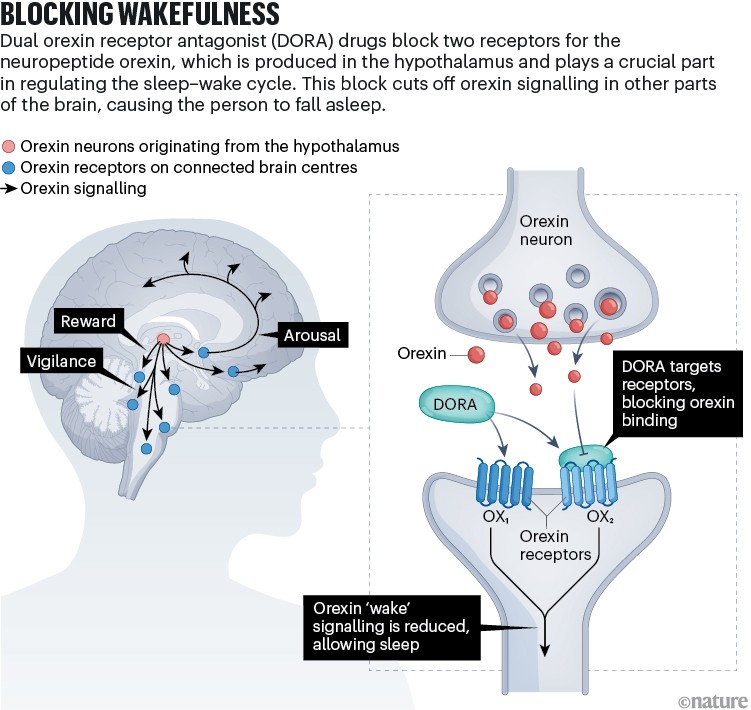
Blocking wakefulness
Mignot was studying narcolepsy, a chronic disorder that affects sleep–wake cycles and causes people to fall asleep suddenly, when he inadvertently helped to pave the way towards the latest means of treating insomnia2. He discovered that dogs with narcolepsy have a genetic mutation that affects one of two receptors used by the neurotransmitter orexin, the primary role of which was initially thought to be the regulation of appetite. Mignot then found that people with narcolepsy lack orexin, confirming the chemical’s main job: promoting wakefulness. If drugs could be developed to prevent orexin from binding to its receptors, Mignot thought, then people with insomnia would become “narcoleptic for one night”.
In 2007, researchers at the pharmaceutical firm Actelion (part of which is now Idorsia Pharmaceuticals in Switzerland) showed that blocking orexin’s two receptors induced sleep in rats, dogs and people3. In 2014, the multinational firm Merck, received US Food and Drug Administration (FDA) approval for the first dual orexin receptor antagonist (DORA) drug, suvorexant (Belsomra). In 2019, another DORA drug — lemborexant (Dayvigo) — was approved, followed, in 2022, by daridorexant (Quviviq).
Compared with benzodiazepines and Z-drugs, which inhibit activity all over the brain, DORA drugs affect only the neurons activated by orexins (see ‘Blocking wakefulness’). “The beauty of it is it does nothing but block the stimulation of wakefulness,” says neurologist Joe Herring, who heads neuroscience clinical research at Merck in Rahway, New Jersey. “It’s a physiologically better way to promote sleep.”
Credit: Alisdair Macdonald
Daridorexant is the only DORA drug for which data are available about daytime functioning, says Antonio Olivieri, chief medical officer at Idorsia, which produces daridorexant. In clinical trials4, Idorsia showed that, compared with those given a placebo, people who received daridorexant experienced significant improvements in daytime insomnia symptoms the following day. Data reported in the approvals database of the FDA also indicate that daridorexant has the lowest fatigue and drowsiness scores of the three DORA drugs, possibly because it leaves the body the quickest.
So far, there have been no one-to-one comparisons of DORA drugs. “Ideally, you’d have direct evidence of how those drugs compare to each other,” says Daniel Buysse, a sleep scientist at the University of Pittsburgh School of Medicine in Pennsylvania. “But we rarely have such evidence, so instead, we have to rely on statistical techniques that allow you to make indirect comparisons.” It’s also difficult to say definitively how DORA drugs compare with older treatments for insomnia, but Buysse says that drug registration trials suggest that DORA drugs have fewer adverse cognitive or hangover effects compared with benzodiazepines and Z-drugs, as well as less potential for dependence and misuse. The European Insomnia Guideline 20235 placed daridorexant as the next recommended insomnia treatment after CBT-I.
The main drawback to DORA drugs, Buysse says, is not medical but financial: their high cost keeps them out of reach of many people who could benefit from them. “There are many patients I would like to prescribe these drugs for, but I know in order for them to get one of these medications we’ll have to go through trials of several other drugs before the request will be considered,” Buysse says. DORA drugs are also available only in a few countries, so far.
Given her long history of insomnia, Miranda was given a prescription for suvorexant. Her psychiatrist recommended the drug to her about a year ago. “I was really sceptical that an anti-wakefulness drug would be any different to a pro-sleep drug,” she says. But she quickly felt the difference, and has now come to see the drug as “a saviour”. Without the drug, she says, “I’d probably be on a much higher benzodiazepine dose than I am.” She hopes her suvorexant dose can continue to increase, so that some of her other medications can be reduced.
Expanding availability
Other drugs that target the orexin system are in the clinical pipeline. Seltorexant, for example, is being developed by the US pharmaceutical firm Johnson & Johnson for people with both major depressive disorder and insomnia. Around 70% of people with depression have insomnia, so having a medication that treats both of those disorders “has the potential to fill an important gap”, says Krystal, who has consulted for Johnson & Johnson on the drug. In a phase III trial6, participants who took the drug experienced meaningful improvement in both sleep and depressive symptoms, with an antidepressant effect that seemed to be independent of the participants getting better sleep. Seltorexant might have an antidepressant effect because it is designed to block only one of the two types of orexin receptor, Krystal adds, whereas other DORA drugs block both receptor types.
Investigations of already-approved DORA drugs are also expanding into other populations. Merck has sponsored investigator-led studies of suvorexant in people with insomnia as well as depression or substance-use disorders, and Idorsia is sponsoring studies of daridorexant’s safety and efficacy in sub-groups of people who have insomnia and other conditions.
In 2020, suvorexant became the first medication to be approved for treating sleep disorders in people with Alzheimer’s disease7. Insomnia is often a precursor to and co-morbid with Alzheimer’s, and the disease seems to manifest differently in people with the condition. In one study8 comparing older people with insomnia with those with both insomnia and Alzheimer’s, people with both conditions had a number of extra changes to their sleep patterns, including less time spent in deep sleep — sometimes called slow-wave sleep because that describes the pattern of the brain’s electrical activity during these intervals. Sleep problems in people with Alzheimer’s also seem to have a causal role in increasing levels of toxic substances in the brains of those individuals. Preliminary data suggest that suvorexant could also help to reduce toxic brain proteins. The results of a follow-up study testing that finding are expected in 2026.
In the weeds
Sleeplessness is already among the most common conditions for the medicinal use of the drug cannabis. Miranda, for example, supplements her nightly pharmaceutical regimen with a cannabis tincture that contains a few of the plant’s 100-plus cannabinoids (she lives in a state where cannabis use is legal). “It’s definitely a key player in my sleep-medication arsenal,” she says.
Yet, scientifically, little is known about which cannabinoids — if any — promote sleep, and what a safe and effective dose is. “Tens of millions of people around the world are probably using cannabinoids for insomnia, but we have very little good-quality evidence to support that,” says Iain McGregor, director of the Lambert Initiative for Cannabinoid Therapeutics at the University of Sydney in Australia.
McGregor is investigating cannabinol (CBN), a molecule that develops in cannabis as the psychoactive component tetrahydrocannabinol (THC) oxidizes. His group reported that CBN increased sleep in rats to a similar degree as zolpidem, but without the drug’s known negative side effect of suppressing rapid-eye-movement sleep9. Unpublished data of a single-night trial with 20 people with insomnia disorder show that people fell asleep 7 minutes faster after taking 300 milligrams of CBN compared with those taking a placebo; participants also reported subjective improvements in sleep and mood. Although 7 minutes “doesn’t sound like a lot”, it is on a par with what benzodiazepines and Z-drugs typically accomplish, says Camilla Hoyos, a sleep researcher at the Woolcock Institute of Medical Research in Sydney, who led the work. McGregor, Hoyos and their colleagues are aiming to follow up the work with a large, community-based trial in which people with insomnia take either CBN or a placebo for six weeks at home.
As for cannabidiol (CBD) and THC — the most well-known cannabinoids — the prospects for efficacy against insomnia are doubtful, at least for the doses used in trials so far. Several small studies have failed to find a sleep benefit from taking CBD. In one experiment10, researchers observed that participants in a study who received 10 milligrams of THC and 200 milligrams of CBD actually slept for 25 minutes less compared with when they received a placebo. Several other company-sponsored trials of low-dose CBD for insomnia were not published, McGregor adds, because they found no significant improvement. “It’s been one failure after the next,” he says.
Insomnia’s new frontiers
The search for more effective insomnia treatments continues in other realms, as well. Some research groups are experimenting with different receptors that they hope could lead to new classes of drugs. Gabriella Gobbi, a clinical psychiatrist and research neuroscientist at McGill University in Montreal, Canada, for example, has homed in on one of the brain’s two melatonin receptors, MT2. “We want to find an alternative mechanism without any addiction liability and with fewer side effects, especially for use in children and elderly people,” she says. A molecule that the team developed that binds to MT2 increased the time that rats spent in deep sleep by 30%11. Gobbi aims to launch clinical trials in the next two to three years.
More from Nature Outlooks
A few companies and health systems, including the US Department of Veterans Affairs and the Cleveland Clinic in Ohio, have also created or are developing digital platforms for delivering CBT-I. These apps take users through regimens that are tailored to their symptoms. SleepioRx, for example, is a 90-day digital programme that has been evaluated in more than two dozen clinical trials and has showed efficacy as high as 76%. This includes helping people to fall asleep faster, sleep better throughout the night and feel better the next day. In August 2024, the programme, developed by Big Health in San Francisco, California, received FDA clearance. A 2024 meta-analysis of 15 studies that compare in-person and electronically delivered CBT-I concluded that the two approaches were equally effective12.
Uptake among physicians has been slow so far, Krystal says. But once practitioners catch on, he adds, “I can imagine a world where you have digital care as your first stop, and if that’s not successful, you see a therapist.”
Some studies suggest that insomnia can stem from a high level of underlying brain activity during sleep. This raises the question of whether reducing this activity could treat insomnia, says Ruth Benca, a psychiatrist at Wake Forest School of Medicine in North Carolina. Companies and academic research groups are beginning to test this proposition with wearable devices that use auditory tones or mild electrical stimulation to increase slow-wave activity in the brain. Some devices are already on the market, and evidence suggests that they can increase the duration of deep sleep. Last June, for example, researchers at Elemind Technologies in Cambridge, Massachusetts, confirmed that auditory stimuli delivered in sync with specific brain-wave rhythms generated in a headband allowed people who usually struggle for more than 30 minutes to fall asleep to shave an average of 10.5 minutes off that time13.
In the coming years, according to Benca, researchers hope to learn enough about insomnia’s causes and treatments to be able to recommend personalized therapies based on an individual’s specific demographics, genetics and co-morbidities. These are the frontiers people are working at, she says.
Even after a lifetime of struggling to find safe and effective help, Miranda says that she still holds out hope that better treatments for insomnia are on the horizon. “I can’t be on these medications forever,” she says. “They’re going to take years off my life.”






